SMA® Alfamino®
Hypoallergenic amino acid-based formula (AAF) with for effective first-line symptom relief in infants and young children with severe CMPA, Multiple Food Protein Allergy and other conditions requiring an AAF.
For complete nutritional support from birth or supplementary feeding from 6 months and up to 3 years of age
Preparing SMA® Alfamino® is very similar to how you prepare standard infant formula. The step-by-step instructions on how to prepare SMA® Alfamino® are:

STEP 1 Wash hands before preparation.
STEP 2 Wash hands before preparation.

STEP 3 Sterilise equipment (by boiling for 5 minutes or with a cold water sterilising solution).

STEP 4 Boil drinking water for 5 min, allow to cool for no more than 30 minutes.
STEP 5 Pour exact amount of water into bottle.
STEP 6 Add exact number of level scoops for age of babyas per feeding table on tin.
STEP 7 Shake bottle until powder fully dissolved. Use immediately and do not keep unfinished bottle, discard contents.
STEP 8 Close tin tightly after each use and store in a cool, dry place. Must be used within 3 weeks of opening.
WARNING “Unboiled water, unsterilsed bottles or incorrect dilution can make your baby ill. Incorrect storage, handling, preparation and feeding can also lead to adverse effects. Follow instructions carefully.”
| AGE | QTY PER FEED (ML) | QTY PER FEED (SCOOPS) | FEEDS PER DAY |
|---|---|---|---|
|
1-2 weeks
|
90
|
3
|
6
|
|
3-4 weeks
|
120
|
4
|
5
|
|
2nd month
|
150
|
5
|
5
|
|
3-4 months
|
180
|
6
|
5
|
|
5-6 months
|
210
|
7
|
5
|
|
From 7th month**
|
210
|
7
|
3-4
|
Glucose syrup, vegetable oils (sunflower, rapeseed, structured palm oil), amino acids, MCT, starch, minerals (calcium glycerophosphate, potassium chloride, sodium citrate, calcium citrate, potassium citrate, sodium phosphate, magnesium oxide, ferrous sulphate, zinc sulphate, copper sulphate, potassium iodide, manganese sulphate, sodium selenate), emulsifier (E472c), Fibre(2'-Fucosyllactose, Lacto-N-Neotetraose), mortierella alpina oil (ARA), choline bitartrate, Schizochytrium sp. oil (DHA), vitamins (C, E, niacin, pantothenic acid, riboflavin, A, thiamin, B6, folic acid, K, D, biotin, B12), acidity regulator (E330), taurine, inositol, L-Carnitine.
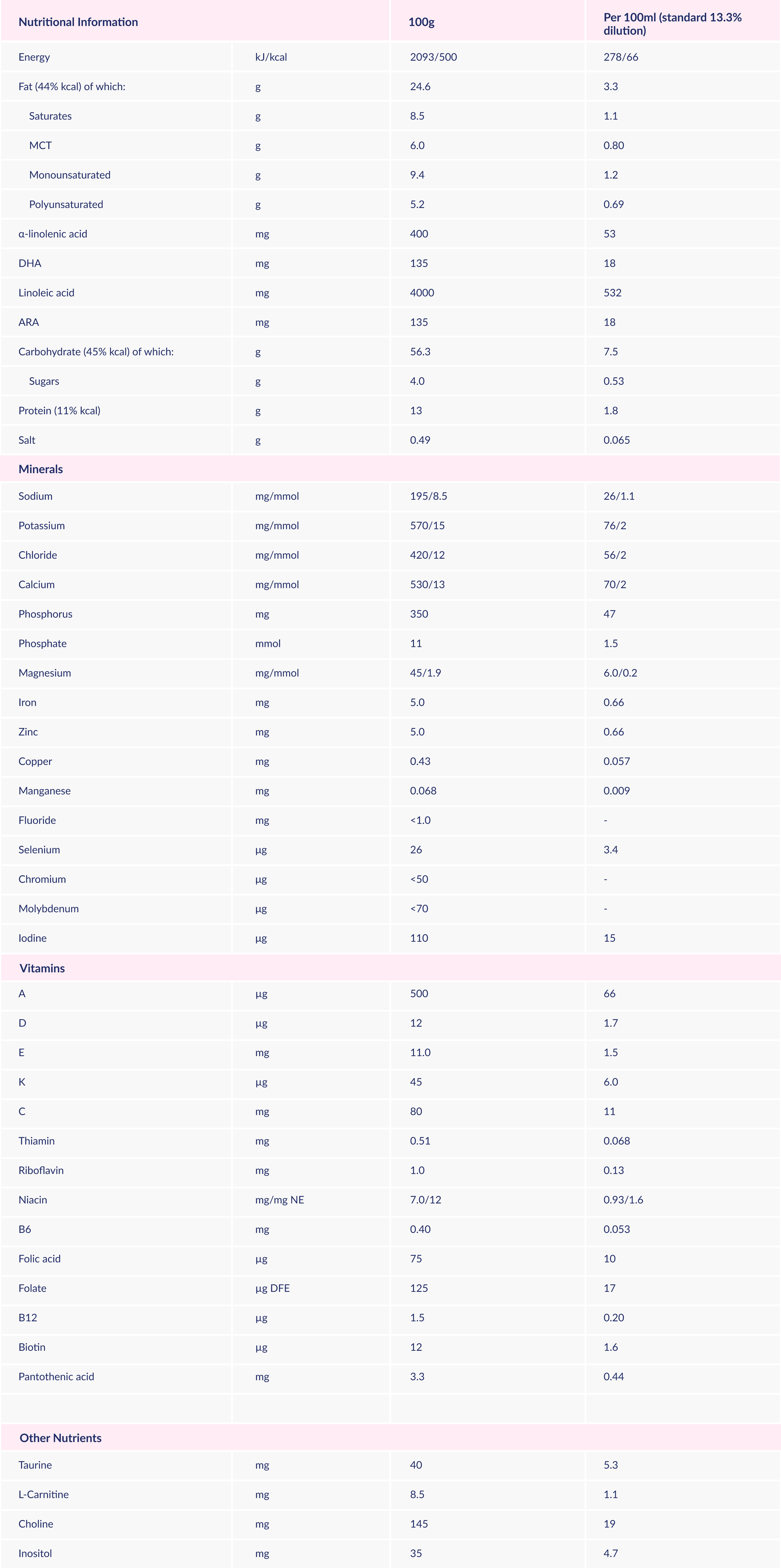
Osmolarity = 294 mOsm/l
Osmolality = 327 mOsm/kg
MCT = Medium Chain Triglycerides
NE = Niacin equivalent
DFE = Dietary folate equivalent
DHA = Docosahexaenoic acid
ARA = Arachidonic acid
- Proven hypoallergenic with a tolerance of 100% in infants and children with Cows' Milk Protein allergy (CMPA)1
- Supports growth and is well tolerated2-4 including in those with severe forms of CMPA4,5
- Inspired by breastmilk with structured lipids (40% in sn-2 position) - associated with higher bone mineral density and better stool consistency7-10
- Designed to support tolerance and absorption with 24.4% MCT11
- Promotes acceptance/compliance with preferred taste over some other amino-acid-based formula12
Patient Case Example For SMA® Alfamino®
NAME & AGE
Sophia, 6 months old
DIAGNOSIS
Sever Cow’s Milk Protein Allergy
DIETARY MANAGEMENT
SMA® Alfamino®

Case presentation: SOPHIA
Sophia, a 6-month-old girl – with severe atopic dermatitis and faltering growth – she had not achieved symptom resolution on an extensively hydrolyzed formula (eHF). She had been exclusively breastfed for the first 4 months and had dry skin from birth, which worsened when she started standard infant formula. She was prescribed emollients and was changed to an eHF at 5-months by her general practitioner (who suspected allergy). This resulted in some improvement, but she continued to have poor growth. She was referred to a dermatologist for assessment.
Atopic Dermatitis (AD) with Faltering Growth (fall of 1 SD from birth to 6 months – birth weight 3.7kg weight-for-age z-score (WAZ) 0 = normal, at 6 months weight 5.7kg, WAZ -2), and cow’s milk protein allergy (CMPA).
The dermatologist prescribed topical corticosteroids and referred her to a dietician.
The dietician recommended changing from the eHF to an amino-acid-based formula (AAF) (SMA® Alfamino®) due to her poor growth and severe AD and proposed a review at 8 months of age. The dietician also provided advice on dairy free complementary feeding.
Sophia’s weight had improved to 7.2kg, WAZ close to 0 at 8 months. Her skin was much better but mum reported flare-ups with certain foods – including wheat and fish, so she was excluding these from Sophia’s diet, along with dairy and soy. They agreed to continue the current diet until her next review when they would consider food challenges.
Her dermatologist had advised a reduction in her topical steroids and to continue with emollients.
On review she was growing well (8.7kg, WAZ 0 = normal) and taking ~500ml/day of SMA® Alfamino®. She was eating well and had a varied diet, although mum was still excluding some foods (dairy, soy and fish). She was now tolerating some wheat-based products. The dietician started dairy reintroduction using the milk ladder at home, but mum reported skin flare-up following the fresh milk challenge. Sophia seemed to tolerate small amounts of soy. The dietician advised that she continue to encourage all foods that were tolerated, excluding only fresh milk and fish for now. It was also suggested to gradually wean her off SMA® Alfamino® over a period of 1-2 months (while monitoring her weight). At her follow-up review (15 month), mum reported that Sophia had stopped SMA® Alfamino® and was taking a commercially available soy-based drink with added calcium. She was still avoiding fish and fresh milk, but Sophia now tolerated baked milk products. She was growing well with her WAZ now back to norma (WAZ = 0). She continued to be seen by the dermatologist, although her skin was also much improved.

SMA® Alfamino® FAQs
Answers to common questions
about
SMA® Alfamino®
.
The tin size of SMA® Alfamino® is 400g.
It is 4.4g – so 1 scoop of SMA® Alfamino® is equal to 4.4g of powder.
Yes, SMA® Alfamino® can be used for infants requiring enteral tube feeding. Instructions need to be followed according to local guidance as well as the patient’s individual nutritional management plan.
Suitable for those following a halal diet (halal certified) but is not kosher certified. The protein used in SMA® Alfamino® is based on 100% free amino acids. This formula meets the hypoallergenicity criteria of the American Academy of Pediatrics (AAP) and can be used for the dietary management of infants with CMPA.1
- Nowak-Wegrzyn A, et al. Clin Pediatr. 2015.
Medium-chain triglycerides (MCT) are made up of a mixture of triacylglycerols consisting of saturated fatty acids with a chain length of 6–10 carbons.1-3 MCT are easily absorbed because they are relatively soluble in water.3 Consequently, they are hydrolyzed both faster and more completely than long-chain triglycerides (LCT), the faster action of pancreatic lipase being facilitated by their small molecular weight.3 These benefits appear to be useful for infants with an immature gut or those with intestinal failure.4 MCT therefore provide a rapid energy source.5 Adding MCT to formulas has been shown to be beneficial in severe fat malabsorption, such as intestinal failure.4 Alfaré® HMO, SMA® Alfamino® and Alfamino® Junior HMO contain 40%, 24% and 24% respectively of MCT fats, which may support energy uptake in infants with severe gastrointestinal impairment (including infants with severe enteropathy affecting fat absorption related to CMPA).
The following conditions have been included in a practical evidence based guide1 on the use of AAF:
- Failure on an extensively hydrolyzed formula (eHF)
- Eosinophilic Esophagitis
- Growth faltering, in particular with multisystem involvement (gastrointestinal tract/and or skin) and multiple food eliminations
- Anaphylaxis
- Meyer R, et al. J Allergy Clin Immunol Pract. 2018;6:383-99.
SMA® Alfamino® is based on 100% free amino acids, contains 24% of it’s fat as medium-chain triglycerides (MCT) and is lactose free. These are important attributes for infants and children with severe forms of CMPA and also support those with severe gastrointestinal (GI) impairment. MCT are easily absorbed and are hydrolyzed both faster and more completely than long-chain triglycerides (LCT).1 This has been shown to be useful for infants with GI impairment.2 Meanwhile being lactose free supports those cases with secondary lactose intolerance.
- Bach AC, & Babayan VK. Am J Clin Nutr. 1982;36(5):950-62.
- Delplanque B, et al. J Pediatr Gastroenterol Nutr. 2015;61(1):8-17.
Discover the Full Range of Options.
We’ve developed nutritional formula solutions.
SMA® Althéra®
From birth onwards.



